CRISPR: The Future of Gene Editing and Its Clinical Implications
CRISPR: The Future of Gene Editing and Its Clinical Implications
In a groundbreaking development, CRISPR technology has become the frontrunner in genome editing, surpassing earlier methods like zinc finger nucleases and transcription activator-like effector nucleases. The recent approval by the FDA of the CRISPR-Cas9 drug, exa-cel, marks a significant milestone in clinical applications, particularly for treating sickle cell disease and transfusion-dependent beta thalassemia.Advancements in CRISPR Technology CRISPR technology is diverse, with various methods tailored for specific applications. CRISPR-Cas9, derived from the immune system of Streptococcus pyogenes, acts as molecular scissors to cut DNA at precise locations. Other variants, such as CRISPR-Cas12 and CRISPR-Cas3, offer unique advantages, including shorter guide RNA requirements and extensive DNA removal capabilities, respectively. Meanwhile, CRISPR-Cas13 targets RNA, opening avenues for treating viral infections like influenza and SARS-CoV-2.
Clinical Trials and Therapeutic Potential The approval of exa-cel, developed by Vertex Pharmaceuticals and CRISPR Therapeutics, is based on promising phase 3 trial data, where a majority of patients with sickle cell disease and beta thalassemia showed significant improvement. Other companies, such as Editas Medicine and Beam Therapeutics, are also exploring CRISPR’s potential in treating these blood disorders through innovative approaches like base editing.
Beyond blood diseases, CRISPR is being trialed for urinary tract infections, hereditary transthyretin amyloidosis, and hereditary angioedema. For instance, Locus Biosciences is conducting trials using CRISPR-Cas3 to tackle antibiotic-resistant UTIs, while Intellia Therapeutics is pioneering therapies for genetic diseases using lipid nanoparticles for systemic delivery.
Expanding Horizons: Cardiovascular and Metabolic Diseases CRISPR’s application extends to cardiovascular diseases, with Verve Therapeutics testing gene editing treatments for familial hypercholesterolemia. In type 1 diabetes, CRISPR Therapeutics is exploring gene-edited pancreatic cells to potentially eliminate the need for lifelong immunosuppression.
Challenges and Ethical Considerations Despite these advancements, challenges remain, particularly concerning the high costs of CRISPR therapies and the regulatory frameworks required to ensure safety and efficacy. Ethical considerations, especially those involving heritable genetic changes, necessitate careful oversight.
As the landscape of gene editing evolves, the focus will be on making these transformative therapies accessible and affordable. The original article from Endocrinology Advisor provides a comprehensive overview of these developments, highlighting the potential of CRISPR to revolutionize medical treatments and improve human health outcomes.
More Articles
Getting licensed or staying ahead in your career can be a journey—but it doesn’t have to be overwhelming. Grab your favorite coffee or tea, take a moment to relax, and browse through our articles. Whether you’re just starting out or renewing your expertise, we’ve got tips, insights, and advice to keep you moving forward. Here’s to your success—one sip and one step at a time!
Exploring the Impact of AI on Real Estate
 Artificial Intelligence (AI) is no longer just a buzzword—it is fundamentally reshaping industries worldwide, with the real estate sector being no exception. From leveraging predictive analytics to offering immersive virtual tours, AI is revolutionizing how properties are bought, sold, and managed. By processing vast amounts of data quickly and accurately, AI empowers buyers, sellers, and agents to make more informed decisions, ultimately creating a more seamless property transaction experience.
Artificial Intelligence (AI) is no longer just a buzzword—it is fundamentally reshaping industries worldwide, with the real estate sector being no exception. From leveraging predictive analytics to offering immersive virtual tours, AI is revolutionizing how properties are bought, sold, and managed. By processing vast amounts of data quickly and accurately, AI empowers buyers, sellers, and agents to make more informed decisions, ultimately creating a more seamless property transaction experience.
In this examination, we delve into AI’s transformative impact on the real estate industry and its future implications for property investment and management. This story is inspired by an insightful article from Substack.
Predictive Analytics for Smarter Decisions
AI employs historical and real-time data to forecast market trends, aiding investors and developers in making informed choices. For buyers and investors, AI-powered platforms analyze market conditions, property values, and neighborhood growth trends. Meanwhile, developers benefit from predictive models that suggest optimal locations and property types, ensuring their projects align with market demand.Virtual Tours and Enhanced Customer Experience
AI-driven virtual reality (VR) and augmented reality (AR) tools revolutionize property viewing, allowing buyers to explore homes remotely, thus saving time and reducing effort. Furthermore, AI enhances the virtual staging of properties, enabling potential buyers to visualize various layouts and designs.Chatbots and AI Assistants
AI-driven chatbots and virtual assistants improve customer service by providing immediate responses to inquiries. For agents and brokers, chatbots handle repetitive queries, freeing them to focus on closing deals. Buyers and renters enjoy 24/7 support, including property searches, viewing scheduling, and mortgage estimates.Property Valuation and Pricing
By analyzing market trends, property features, and comparable sales, AI algorithms provide precise property valuations. Sellers can set competitive asking prices, while buyers are ensured to not overpay by having insights into market data and predicted price trends.Fraud Detection and Risk Management
AI enhances security and reduces risks in real estate transactions. It detects fraud by identifying unusual patterns in data and assesses potential risks in investments, such as market volatility or environmental hazards, providing critical insights for investors.Streamlined Property Management
AI-powered tools bring efficiency to property management. Smart maintenance systems predict property conditions and maintenance needs, preventing costly repairs. Additionally, AI evaluates tenant applications, ensuring reliable renters are selected based on credit history and rental behavior.Some noteworthy examples of AI integration include Zillow’s AI-powered algorithms for property valuations, Compass’s use of AI in providing tools for pricing strategies, and OpenDoor’s streamlined home-buying and selling processes.
While AI offers significant opportunities, its widespread adoption faces challenges such as data privacy concerns, bias in algorithms, and the substantial investment required for technology integration. Looking ahead, AI’s role in real estate promises innovations like smart contracts, blockchain integration, and a shift toward AI-driven property development. The technology is expected to contribute to smarter cities, facilitate global real estate transactions, and promote sustainability through optimized energy management in properties.
Divided Nation: Trump’s Second Term Begins with Controversy
Trump’s Second Term: Approval Ratings, Pardons, and Public Opinion
As Donald Trump begins his second term as President of the United States, a recent Reuters/Ipsos poll reveals a deeply divided nation. With an approval rating of 47%, Trump starts this term with a higher popularity than during most of his first tenure. This figure, however, is accompanied by significant discontent regarding some of his initial actions, particularly his decision to pardon individuals involved in the January 6, 2021, Capitol riot.Approval Ratings and Controversial Pardons
The poll, conducted just after Trump’s inauguration, highlights the contentious nature of his pardons. A substantial 58% of respondents opposed pardoning those convicted in connection with the Capitol siege. Despite this opposition, Trump proceeded to pardon nearly 1,600 individuals involved, including 14 leaders of the incident, mere hours into his second term. In contrast to these controversial pardons, Trump’s handling of immigration issues garnered a more favorable response. Approximately 46% of respondents approved of his approach, with many Americans expressing a desire for immigration reform to be prioritized by the new administration. A notable 58% agreed with reducing the number of migrants allowed to claim asylum at the U.S. border, reflecting support for Trump’s early actions to restrict immigration.Polarization and Political Dynamics
Trump’s approval ratings, while higher than those of his first term, remain historically low compared to other U.S. presidents who typically begin their terms with approval ratings above 50%. As political analyst Jacob Rubashkin points out, Trump’s ratings are “roughly in line with what we saw in the first term,” indicating a persistent polarization within the American public. The survey also highlights the stark partisan divide, with 91% of Republicans approving of Trump’s leadership and 84% of Democrats disapproving. This division mirrors the political landscape during Joe Biden’s presidency, which saw similar challenges in garnering bipartisan support.International Ambitions and Public Sentiment
Trump’s return to office brings with it potential shifts in international relations. However, the poll suggests limited public support for his more ambitious plans, such as acquiring Greenland from Denmark or regaining control of the Panama Canal. Only 16% of respondents supported the idea of pressuring Denmark to sell Greenland, and a mere 21% believed the U.S. should expand its territory in the Western Hemisphere. These findings indicate that while Trump may focus on satisfying his core supporters, broader public opinion remains skeptical of his territorial ambitions. As public opinion expert John Geer notes, second-term presidents often prioritize their legacy over popular opinion, suggesting that Trump may continue to pursue policies aligned with his “Make America Great Again” movement.Conclusion and Future Prospects
As Trump navigates his second term, the challenges of maintaining a balanced approach between satisfying his base and addressing broader national and international concerns will be crucial. His initial actions have already sparked significant debate and opposition, but they also highlight the enduring support among his most ardent followers. Moving forward, Trump’s ability to manage these dynamics will likely define the success of his presidency and its impact on both domestic and global affairs. For those interested in following these developments, the Reuters Politics U.S. newsletter offers weekly insights and analysis on how U.S. politics influence the world.Time for a Change? Signs Your Property Management Needs an Overhaul
Time for a Change? Signs Your Property Management Needs an Overhaul
As the year draws to a close, property owners may find themselves reflecting on the effectiveness of their management companies. David Crown, CEO of L.A. Property Management Group, recently shared insights on when it’s time to consider a change in management. His article, published on Forbes, highlights three critical signs that it might be time to seek new management in 2025.
1. Poor Communication and Responsiveness
Effective communication is the bedrock of any successful property management relationship. When communication falters, property owners can feel left in the dark, leading to frustration and distrust. Crown emphasizes that if your current management company frequently ignores calls or emails, it might be indicative of deeper systemic issues. A management team that prioritizes timely, clear, and proactive communication is essential for peace of mind.2. Lack of Modern Technology and Innovation
In an ever-evolving business landscape, the adoption of modern technology is crucial. Crown shares that his company has implemented a new artificial intelligence (AI) platform to automate maintenance scheduling, significantly improving response times. If your current management company hasn’t embraced technological advancements, it may result in slower, less efficient service. AI is more than just a buzzword; it’s a transformative tool in property management.3. High Tenant Turnover Rates
High tenant turnover can be costly and disruptive. It often signals tenant dissatisfaction due to poor service or unaddressed maintenance issues. Good property management companies focus on tenant retention through responsive service and effective communication. If you’re experiencing high turnover rates, it’s worth investigating the underlying causes.In conclusion, if these signs resonate with your experience, it might be time to explore other management options. Your property is a priority, and ensuring that your management team aligns with your best interests is crucial for long-term success.
For more insights, visit the original article on Forbes.
19 Real Estate Investment Trends to Watch in 2025
 In the ever-evolving world of real estate, staying ahead of the curve is not just advantageous, but essential. As we peer into the horizon of 2025, the landscape is poised to be shaped by a confluence of emerging trends. A recent Forbes Business Council article delves into these anticipated shifts, offering insights from 19 industry experts.
In the ever-evolving world of real estate, staying ahead of the curve is not just advantageous, but essential. As we peer into the horizon of 2025, the landscape is poised to be shaped by a confluence of emerging trends. A recent Forbes Business Council article delves into these anticipated shifts, offering insights from 19 industry experts.
1. Embracing a Growth Mindset
Real estate investors are gearing up for 2025 with an increased spending on both new and existing properties. This proactive approach, highlighted by RentRedi‘s Ryan Barone, suggests a diversification of portfolios and a keen interest in geographical and property type expansion. Such strategies are expected to bolster the rental property sector and open doors to new markets.2. Demand for Flexible and Sustainable Spaces
The hybrid work model is driving a surge in demand for flexible spaces in prime locations, as noted by Beate van Loo-Born of PhysikInstrumente (PI). Coupled with this is a growing emphasis on sustainability, with investors and tenants increasingly prioritizing eco-friendly and resilient buildings.3. Navigating High-Risk Areas
Nathan Miller from Rentec Direct anticipates a strategic shift away from high-risk regions, such as the hurricane-prone Southeast and wildfire-vulnerable Northwest. This creates opportunities for investors with a higher risk tolerance and may present attractive prospects for first-time homebuyers.4. Technological Advances in Real Estate
The integration of AI-driven property analyses is set to revolutionize the industry by 2025. As Shehar Yar of Software House explains, leveraging predictive analytics will enable investors to identify high-yield opportunities with precision, although it may also heighten competition and inflate property prices in hotspots.5. The Rise of Eco-Friendly Investments
Stephen Nalley from Black Briar Advisors foresees a surge in demand for eco-friendly real estate, driven by climate awareness and sustainability incentives. Investors focusing on green properties may reap higher returns as tenants and buyers increasingly seek energy-efficient spaces.Additional Trends to Watch
- Increased social impact investing, particularly in affordable housing, as discussed by Seth Gellis of Community Preservation Partners.
- Growth in co-living spaces, catering to those seeking affordable and flexible living arrangements, highlighted by Goro Gupta of Ethical Property Investments.
- Investment in digital infrastructure, such as data centers and server farms, noted by Sabeer Nelliparamban of Tyler Petroleum Inc.
These insights from the Forbes Business Council underscore the dynamic nature of the real estate market. As 2025 approaches, investors are encouraged to stay informed and agile, adapting to these trends to optimize their strategies and capitalize on emerging opportunities. “`
AI Outperforms Doctors in Diagnostics but Faces Challenges in Clinical Integration
LLMs: The Future of Diagnostic Accuracy?
The study meticulously compares the diagnostic reasoning of physicians using conventional resources against the standalone performance of LLMs. It reveals a stark contrast: while LLMs independently deliver superior diagnostic results, their integration into clinical practice requires strategic enhancement to complement, not replace, human expertise.AI as a Supplementary Tool
Despite the impressive diagnostic capabilities of LLMs, the study emphasizes their role as supplementary tools in healthcare settings. The integration of these AI models should aim to augment the expertise of physicians, ensuring that human judgment remains central to patient care. This approach calls for comprehensive training for healthcare professionals to effectively utilize LLMs, optimizing their performance through structured prompt design.
Challenges and Considerations
The findings suggest a nuanced approach to AI integration in clinical settings. While LLMs demonstrate remarkable diagnostic accuracy, their role should not undermine the indispensable aspects of human interaction and judgment in medical practice. As AI technology continues to evolve, the healthcare industry must prioritize patient care by leveraging these tools to enhance, rather than overshadow, the expertise of medical professionals.Looking Ahead
The study’s conclusions highlight the need for ongoing research and evaluation of AI applications in healthcare. As LLMs inch closer to clinical integration, it becomes imperative to develop reliable metrics and evaluation methods that reflect real-world clinical scenarios. This will ensure that AI tools are used to their fullest potential, enhancing diagnostic reasoning while safeguarding patient welfare.The Role of Blockchain in Real Estate
The Role of Blockchain in Real Estate
The transformative power of blockchain technology is making significant waves across various industries, and real estate is no exception. Once considered a novelty, blockchain in real estate is now a practical solution revolutionizing the sector. As highlighted in a recent Appinventiv article, blockchain offers a myriad of applications beyond cryptocurrencies, providing innovative solutions to the industry’s prevailing challenges.Challenges in Real Estate The real estate industry has long been plagued by several inefficiencies, including a lack of transparency, tedious paperwork, higher risk of fraud, expensive investments, and poor transaction speed. These issues have historically hindered the sector’s growth and accessibility. However, blockchain technology offers a beacon of hope, addressing these challenges head-on.
Blockchain Solutions Blockchain technology introduces transparency through decentralized records, reducing the need for intermediaries, and enhancing transaction efficiency with smart contracts. Smart contracts automate processes, eliminating the need for middlemen, and ensuring transactions are executed when specific conditions are met. This not only speeds up transactions but also reduces the risk of human error.
Moreover, blockchain facilitates asset tokenization and fractional ownership, making real estate investments more accessible by allowing investors to purchase and sell fractional shares of properties. This democratizes real estate investing and enhances market liquidity.
Global Accessibility and Efficiency Blockchain’s decentralized infrastructure significantly enhances global accessibility, enabling seamless cross-border transactions. This opens up the real estate market to a broader range of investors, promoting diversification across different geographical areas. Furthermore, by eliminating intermediaries and automating processes, blockchain reduces administrative costs and accelerates transaction speeds, making real estate transactions more efficient and cost-effective.
Real-World Applications Several major players in the real estate industry have already begun leveraging blockchain technology to streamline operations and offer innovative investment opportunities. For instance, CBRE Group is using blockchain to transform property management, while Simon Property Group utilizes it for tenant relations and retail lease administration. These applications set new standards for efficiency, transparency, and tenant involvement in the real estate sector.
Overcoming Challenges Despite its potential, the integration of blockchain in real estate is not without challenges. Issues such as inadequate knowledge, scaling, and chain interoperability pose significant hurdles. However, solutions like education and training programs, collaboration with blockchain development firms, and the adoption of interoperability protocols can help overcome these barriers.
Conclusion The future of real estate is poised for a revolutionary change with the integration of blockchain technology. As businesses continue to explore and implement blockchain solutions, the real estate industry is set to become more transparent, secure, and efficient, paving the way for a more inclusive and reliable market landscape.

48th Edition of Florida Real Estate Pre-License Textbook: A Must-Have Resource
For aspiring real estate professionals in Florida, obtaining the right educational resources is crucial to success. The 48th edition of the Florida pre-license real estate textbook, entitled “Textbook for Sales Associate Pre-License Course- Florida Principles, Practices & Law”, is a pivotal tool for those preparing for their real estate exam. This newly updated edition for 2025 is specifically tailored to meet the needs of students entering the dynamic world of real estate.
Available at the Florida Real Estate Book Store, this textbook is priced at $60.00, making it an affordable investment in your future career. The book is designed to provide comprehensive coverage of essential real estate principles, practices, and laws specific to Florida. It serves not only as a study guide but also as a valuable reference throughout your real estate career.
While the specific details of the 48th edition are not extensively covered in other sources, it is clear that the updated content reflects the latest changes in real estate laws and practices, ensuring that students are well-prepared for both the exam and real-world applications. The textbook is a part of a broader educational offering that includes various real estate courses and materials aimed at providing a solid foundation for students. You can explore further options related to real estate textbooks at the Florida Real Estate Book Store or check out additional resources and course materials at Florida Real Estate School Courses and Products.
For those considering a career in real estate, it’s important to stay informed about the best resources available. Utilizing the latest edition of the textbook ensures that you are up-to-date with current industry standards and regulatory requirements. Additionally, engaging with supplementary resources such as practice exams, online courses, and study groups can significantly enhance your learning experience.
In conclusion, the 48th edition of the Florida pre-license real estate textbook is an indispensable resource for any aspiring real estate professional in Florida. By investing in this updated edition, you are taking a critical step towards achieving success in your pre-licensing course and beyond. Make sure to visit the Florida Real Estate Book Store to secure your copy and embark on your journey with the confidence that you have the most current and comprehensive materials at your disposal.
Gene Editing: Hope, Hype, and Humanity’s Ultimate Challenge
One of the most controversial chapters in this saga was written by He Jiankui, a Chinese biophysicist who, as reported in the MIT Technology Review, created the first “CRISPR babies” with the aim of making them immune to HIV. This bold move led to his imprisonment and sparked a global debate on the ethics of genetic manipulation.
The Ethical Quagmire
The implications of editing the human genome are profound. As highlighted in the original article, the potential to change human evolution raises questions about morality and the long-term effects on our species. Gene editing in embryos, which is restricted or illegal in many parts of the world, could lead to a future where genetic enhancements are commonplace, potentially creating a new form of inequality.Scientific Perspectives
Experts like George Church and Fyodor Urnov offer varied insights into the future of genetic editing. Church envisions a world where enhancements are as common as consumer technology, while Urnov warns of the potential for misuse and the ethical dilemmas that accompany such power. The article also references He Jiankui’s announcement of his intention to continue research, albeit with more caution.
The Road Ahead
As technology continues to advance, the line between enhancement and therapy becomes increasingly blurred. The biotechnology industry is already exploring ways to emulate beneficial genetic variants, potentially offering enhancements to those who can afford them. This raises concerns about accessibility and fairness, as well as the potential for unforeseen consequences.The future of human evolution, as discussed in the article, may not rely solely on editing embryos. Instead, advances in delivering CRISPR technology to adults could democratize genetic enhancements, making them available to a broader population. However, this also opens the door to new risks, including the possibility of unauthorized genetic modifications.

Conclusion
The potential to rewrite the human genome is both exhilarating and daunting. As we stand on the brink of a new era in biotechnology, the choices we make today will shape the future of our species. The conversation around gene editing is far from over, and it remains to be seen whether humanity will wield this power with wisdom and responsibility.Unveiling the Impact of 3D Printing on Business Growth
Unveiling the Impact of 3D Printing on Business Growth
In today’s rapidly evolving technological landscape, 3D printing emerges as a revolutionary force propelling businesses towards sustainable growth. This transformative technology not only addresses pressing environmental concerns but also reshapes production methods across industries, enhancing efficiency and enabling innovation.3D printing significantly reduces material waste and promotes on-demand production, proving invaluable in industries like healthcare, automotive, and aerospace. Notably, startups like rrreefs and Impressora are utilizing 3D printing to craft eco-friendly solutions, such as artificial coral reefs and affordable housing, respectively.
The Versatile Applications of 3D Printing
Exploring the myriad ways 3D printing fosters business innovation, the original article from StartUs Insights highlights:
- Tackling Climate Change: By minimizing energy use and waste, 3D printing supports sustainable manufacturing practices. Companies like rrreefs are developing 3D-printed structures to combat climate change and rejuvenate marine ecosystems.
- Navigating Demographic Shifts: The technology allows for customized healthcare solutions, such as personalized prosthetics and implants. Viortec, for instance, enhances joint replacement surgeries with tailored 3D-printed implants.
- Rapid Urbanization Solutions: Efficient, cost-effective housing solutions are possible through 3D printing. Companies like Impressora integrate eco-friendly materials in constructing modular and customizable urban infrastructure.
- The Energy Transition: In energy sectors, 3D printing aids in producing optimized components, like custom wind turbine parts, enhancing energy efficiency and sustainability.
- Emerging Trends in Mobility: 3D printing facilitates the production of lightweight vehicle components, improving fuel efficiency and enabling swift prototyping, pivotal for innovative mobility solutions.
Startups Revolutionizing Industries
Several startups harnessing 3D printing are highlighted, showcasing its diverse applications in addressing global challenges. Examples include LPrint for hyper-connectivity via printed electronics and Additive Drives GmbH for advancing electric motor technology in transportation.
The Outlook for 3D Printing
The innovative future of 3D printing is underscored by continuous investments and technological advancements, with significant contributions from industry leaders like Techstars and MassChallenge. The technology’s global footprint is expanding, with major hubs in the USA, Germany, and beyond, indicating a promising trajectory for industry growth.
Conclusion
3D printing stands as a pillar of innovation, driving industries toward a sustainable and efficient future. With its ability to address global economic, environmental, and societal shifts, 3D printing redefines possibilities across sectors, marking a new era of industrial advancement.
10 Essential Real Estate Concepts from Prep Agent’s Crash Course
10 Real Estate Concepts You Need to Know: My (Slightly Overwhelmed) Reaction to Prep Agent’s Crash Course
Alright, imagine this: you want to become a real estate mogul—or maybe you just need your real estate license so you can finally stop fantasizing about flipping that shady duplex down the block. Either way, you’ve got to pass your real estate exam. And if, like me, you’ve ever felt the creeping doom of important information flying over your head, then Joe from Prep Agent is absolutely your guy.
Joe’s latest breakdown of “10 Concepts You Must Know to Pass Your Real Estate Exam” feels like drinking from a firehose—but in the best way possible. Picture a no-nonsense friend who drags you through all the need-to-know basics, but does it with the tough love of a coach who really wants you to win—and maybe yell “studs” under your breath at practice.
So, buckle up. I’m here to unpack Joe’s crash course in a somewhat digestible (and hopefully entertaining) way while processing how I, too, might survive this mental workout.
Real Property vs. Personal Property: What’s Planting Roots and What’s Hitting the Road?
First up, Joe hit us with the concept that real property is immovable (think land, buildings, the roots of your sanity), while personal property is movable (shoes, maybe your coffee maker if you’re civilized, or even your lease agreement).
“Real property goes to the REAL estate; personal property goes with the PERSON.”
Easy enough, right? Except now I’m looking at my potted plant wondering if I’d have the emotional bandwidth to let it go during a sale. (Spoiler: I wouldn’t. It’s coming with me. Thanks, Joe.)
Estates: Freehold, Not-So-Freehold, and Deadbeat Tenants
Let’s talk estates. Apparently, there are freehold estates (aka you own it forever) and less than freehold estates (leases that come with expiry dates). The part that stuck in my brain like peanut butter? Joe calling tenants who overstay their welcome a “deadbeat tenant.” Honestly, iconic.
- Estates for years (think a summer rental)
- Periodic tenancy (month-to-month rentals)
- Estate at will (a wildcard lease that could poof into thin air)
- Estate at sufferance (translation: “Please leave; you’re here too long.”)
Freehold estates, on the other hand, are where the real drama lives—are you sipping a life estate or skipping alcohol sales on your property because of a weird condition? Don’t worry; Joe’s got you covered.
P.E.T.E. the Power-Hungry Uncle: Government Powers
When Joe mentioned P.E.T.E., I immediately imagined a guy at Thanksgiving who constantly chimes in with unsolicited advice (and occasional ultimatums). P.E.T.E. is all about government powers:
- Police Power: “You can keep your home, but you will follow zoning laws.”
- Eminent Domain: “We’re taking this for a freeway, but here’s a check.”
- Taxation: Pay the man.
- Escheat: No heirs? Your property goes to the state.
PETE doesn’t mess around.
Ownership: Are You Flying Solo or Part of a Real Estate Squad?
Here’s where joint tenancy and tenancy in common entered the chat. If you’re into acronyms, joint tenancy sounds like #SquadGoals: T-TIP (time, title, interest, and possession shared equally). If one buddy kicks the bucket, the others absorb their share like some kind of financial photosynthesis. With tenancy in common, however, everyone gets their own slice of the pie. Die? Your slice goes to your heirs. A tidy way of saying, “You do you, boo.”
S.T.U.D. (or D.U.S.T.): Essential Elements of Value
Scarcity, Transferability, Utility, and Demand. Without these, your property value might as well be imaginary.
For instance, being the only house on an island (scarcity) = cha-ching. Living behind an airport (low utility)? Maybe not so much.
Depreciation: When Stuff Falls Apart
- Economic obsolescence: External problems (e.g., neighbors with backyard chickens).
- Functional obsolescence: Bad designs (e.g., no bathrooms in your 10-bedroom house).
- Physical deterioration: Your house is straight-up falling apart.
The Market Data Approach vs. The “How Much Do Shoes Cost?” Method
Fair pricing boils down to:
- Market Data Approach: It’s like saying, “These sneakers cost $100 at three stores, so I guess that’s the fair price!”
- Cost Replacement Approach: Replacing the structure piece by piece.
- Income Capitalization Approach: How much rental income will this generate?
Special shoutout to libraries, schools, and police stations for transcending traditional valuation metrics. We see you.
Deeds vs. Titles: The “Marriage Certificate” of Real Estate
Deed: Proof of ownership transferring.
Title: Actual ownership.
Simple. Just don’t confuse it with a marriage certificate, which is…well, another story.
Fair Housing Laws: Don’t Steer, Blockbust, or Redline—Ever
Joe wrapped up strong with concepts that everyone (not just future agents) should know:
- Steering: Don’t guide buyers based on race or ethnicity.
- Blockbusting and panic selling: Big no.
- Redlining: Drawing circles to exclude areas from lending? Hard pass.
This isn’t just about the exam—these are the basics of being a decent human being who understands 1968 was a pivotal year.
Let’s Hear It for Joe…and the Hustle!
I’ve gotta hand it to Joe—he didn’t just outline 10 real estate concepts; he threw in memory hacks (thank you, T-TIP and S.T.U.D.), dad jokes (here’s looking at you, “deadbeat tenants”), and the kind of brutally honest perspective I personally find refreshing.
Seriously, if you’re prepping for the exam or just curious about dipping your toes into real estate, Joe’s content lays a solid base—even if your brain feels like mush afterward.
What about you? Are you knee-deep in real estate study prep or just mildly intrigued by all the acronyms? Share your experiences in the comments below. And hey, don’t forget: real property stays; personal property goes. That’s advice for real estate and life.
Beyond the Vocabulary Drill: Jonathan Goforth’s Real Estate Prep
Ever Wondered Why Real Estate Exams Feel Like Brain Surgery?
Let’s face it—studying for the real estate exam feels like being force-fed a dictionary while simultaneously dissecting a Sudoku puzzle. After trawling through Jonathan Goforth’s marathon YouTube transcript—a treasure trove of real estate practice questions—I found myself flinching at words like “alienated,” “hypothecated,” and “impertinence.” Who knew passing the real estate exam felt like acing a spelling bee for vocabulary you’ll never use again (yes, I’m looking at you, “severance”)?
But hey, if reaction content is your preferred dose of study procrastination, you’re in for a treat. Consider this article your much-needed coffee break—a relatable, digestible analysis of Jonathan’s 75-best-question classroom. Let’s dive in, shall we?
Context: What Is Jonathan’s Video Even About?
Picture this: Jonathan Goforth (yes, that’s really his name—does fate truly exist?) sits down with us eager learners to share 75 handpicked real estate exam questions. By “handpicked,” I mean he plucked them from previous videos because, well, when you’ve got content, why not cobble together a highlight reel? He promises these questions work for all 50 states—which is comforting, unless, like me, you’re bad at math anywhere.
He wants us to master concepts like leasehold estates, deed restrictions, and the strange world of easements (spoiler: it’s way less exciting than it sounds). But wait, there’s more—he includes tips on overcoming obnoxious test trickery and the dreaded double negatives. You know the drill: “Which of the following is not unessential?” Um… what?
By the end of it, you’re ready to scream, “Yes, Jonathan, I will screenshot every slide and pass this test just so I can stop hearing the word ‘encumbrance’ in my nightmares!”
Main Reaction: Confusing Yet Weirdly Addictive
The Good
- Instant Clarity on Tough Concepts: It’s like he grabbed that bowl of spaghetti noodles you call knowledge and untangled it. For example:
- Appurtenances: Sounds like a Hogwarts spell but actually means rights or improvements that “run with the land.” Get a pool? Boom, appurtenance. Mineral rights? Yup, that too.
- Encumbrances: These are party poopers like liens and easements that burden property titles. Got a fence? Sorry, that’s not “easy, breezy, beautiful” either.
- Cheat Sheet Tips for Math Haters: Jonathan gives you simple formulas for stuff like calculating real estate taxes or down payments. Example: “Property sold for $400,000. LTV is 80%. What’s the loan?” Math-phobes rejoice—he shows you exactly when to multiply, subtract, and ignore irrelevant details.
- Realistic Test Scenarios: Jonathan delivers tricky questions you’ll undoubtedly see on your exam. Remember “Which government power does not include condemnation?” (Hint: It’s not listed, because condemnation is a process… mind blown).
The Frustrating
- The Transcript Reads Like a Scroll from the Dark Ages: Clocking in at enough words to rival War and Peace, this script kind of broke me. I scrolled endlessly trying to stay on track. It didn’t help that questions like “Which of the following is not a characteristic of land?” required mental gymnastics just to break down.
- Vocabulary Overload: Severance? Alienation? Hypothecation? I mean, does anyone actually say these things outside the exam? Realistically, you’ll forget 75% of this the second you ace the test. (Trust me, I’ve Googled “tenancy in common” five times already.)
Analysis And Comparisons: Why Jonathan Is The MVP of Real Estate Prep
From YouTube University to cram apps, there are a gazillion ways to prep for the real estate exam. Yet Jonathan’s granular video sticks out because:
- He’s Thorough AF: Unlike TikTokers breezing through ten-second flashcards, Jonathan dives dee-eep. Want to understand why a ceiling fan is personal property until it’s installed (hello, fixture status)? He’s got you covered.
- His Humor is Dry and Dad-Like: Occasionally self-deprecating, Jonathan is like that one teacher who goes rogue on lecture plans but somehow keeps the jokes coming. Example: He reminds us that Realtors rarely use the fancy jargon the test obsessively drills into our heads. A truth bomb I needed.
Engagement: So… What Did YOU Think?
Look, we can’t all be walking encyclopedias like Jonathan. I’m genuinely curious—how are you tackling real estate study stress? Are you screencapping YouTube slides like a digital hoarder? How many practice questions have sent you into a minor existential crisis?
Let’s vent together—leave a comment below! Whether you’re team “memorize the glossary” or team “watch-the-test-prep-guy-three-times-faster,” I genuinely want to hear your study hacks and horror stories.
Mainstreaming Blockchain: A New Era of Finance
Mainstreaming Blockchain: A New Era of Finance
In a world where traditional finance is not just adopting crypto but being fundamentally rebuilt around it, Forbes reports on the seismic shifts reshaping the financial landscape. Major financial institutions are not only embracing blockchain technology but are actively integrating it into their core operations, heralding a new era of financial innovation.
Blockchain Integration in Banking JPMorgan has taken a pioneering step by launching instant dollar-euro conversions on its Kinexys blockchain platform. This move is part of a broader trend where transaction volumes have surged tenfold, processing over $2 billion daily. Meanwhile, Visa’s Tokenized Asset Platform is enabling banks like BBVA to create and manage digital tokens, with pilot programs slated to commence in 2025.
Advancements in Tokenization Tokenization is emerging as a transformative force, bridging traditional finance with the decentralized world. Industry projections suggest that the market for tokenized real-world assets could expand dramatically, potentially reaching $10-15 trillion within the next decade. Goldman Sachs is at the forefront with the launch of three new tokenization products, focusing on money market funds and real estate assets.
AI and Blockchain Collaboration The collaboration between AI and blockchain is forging a new digital frontier. As artificial intelligence decodes complex data patterns, blockchain anchors these insights in an immutable ledger. This synergy is transforming raw information into verifiable, tamper-proof intelligence, rewriting the rules of data integrity. VeraViews exemplifies this innovation by integrating blockchain-based Proof of View technology with AI-driven fraud detection, showcasing how emerging technologies can tackle industry-wide challenges.
The Rise of DeFi and Stablecoins DeFi represents blockchain’s most radical innovation yet—a financial system that operates purely on code. By replacing traditional intermediaries with automated smart contracts, DeFi enables complex financial operations to occur in seconds. Stablecoins, maintaining stable value through fiat currency pegs, serve as the critical bridge between DeFi and traditional finance, accelerating mainstream adoption.
Cross-Border Payments and Technology Innovation Blockchain is revolutionizing cross-border payments, creating new pathways that bypass traditional banking systems. The combination of blockchain networks and stablecoins is transforming the global remittance market, estimated at $630 billion in 2022, by enabling faster and cheaper transfers. To support these transactions, robust exchange infrastructures like BestChange are emerging, providing cryptocurrency exchanger directories that aggregate real-time rate comparisons, contributing to market efficiency and accessibility.
The Path Forward: Regulation and Growth The regulatory environment has matured significantly, with comprehensive frameworks balancing innovation with consumer protection. This clarity is crucial for institutional adoption, providing the certainty needed for larger financial institutions to invest in blockchain-based solutions.
A New Era of Finance As traditional finance integrates digital innovations, it is actively building the future of finance. Those who recognize this shift are not just adapting to change but are positioning themselves to shape how value moves in a digital economy.

AI in Radiology: Balancing Innovation with Concerns
AI in Radiology: Balancing Innovation with Concerns
A recent analysis has shed light on the public’s perception of artificial intelligence (AI) in radiology. While the majority of patients are supportive of AI’s integration into this field, concerns about data privacy and job displacement remain prevalent. The study, led by Mansour Almanaa, PhD, from King Saud University in Saudi Arabia, provides a nuanced view of the current discourse surrounding AI in healthcare.Understanding Public Sentiment
Almanaa’s research involved a comprehensive analysis of over 1,000 social media posts on platforms like Reddit and X (formerly Twitter) spanning from 2019 to 2024. The findings, published in the journal Cureus on September 23, revealed that approximately 55% of comments were positive, highlighting AI’s potential to enhance diagnostic accuracy and efficiency. However, 35% of the comments were neutral, and 10% expressed negative sentiments, primarily focused on job loss, ethics, and privacy concerns.Expert Insights
Mansour Almanaa emphasized the necessity of addressing these concerns to ensure AI’s responsible application in medical imaging. He advocates for the development of clear regulatory frameworks and ethical guidelines to safeguard patient safety and data security. Furthermore, Almanaa underscores the importance of continuous education for healthcare professionals to adapt to AI’s evolving role in medicine.Methodology and Challenges
The study meticulously filtered through nearly 4,000 posts using 20 different search phrases such as “radiology,” “computed tomography,” “AI,” and “medical imaging.” The sentiment analysis was conducted using Python’s VADER tool, focusing on ethical and privacy issues associated with AI. The study identified challenges including the transparency of AI processes and accountability for AI-generated errors.The Dual Nature of AI’s Impact
Despite the concerns, there is recognition of AI’s potential to complement the work of radiologists rather than replace them. AI’s ability to automate routine tasks and improve workflow efficiency could allow radiologists to focus on more complex cases, potentially reducing their overall workload.Conclusion
Almanaa’s study highlights a general acceptance of AI’s benefits in medical imaging but stresses the need to address ethical, privacy, and job displacement concerns. Future research should focus on creating robust ethical standards and regulatory practices, while also supporting continuous education for healthcare professionals to effectively integrate AI into medical practice.For further details, the original article can be accessed here. “`
Predictive Analytics Tools Reshaping Business Landscape by 2025
In the ever-evolving landscape of business technology, predictive analytics tools are emerging as indispensable assets for companies aiming to stay ahead of the curve. As we look towards 2025, these tools are not only becoming more sophisticated but also increasingly accessible to both IT professionals and business users alike.
In a recent article by TechTarget, eight leading predictive analytics tools were profiled, each offering unique capabilities that cater to diverse business needs. These tools are paving the way for more intuitive and efficient data analysis, empowering users to make informed decisions with greater ease.
Revolutionizing Predictive Analytics
The traditional realm of analytics focused on understanding past events through descriptive analytics and diagnostic analytics. However, predictive analytics shifts the focus towards forecasting future outcomes by examining patterns and trends in data. This transformation has been accelerated by advancements in automated machine learning (AutoML), which simplifies the predictive modeling process.
According to Carlie Idoine, a vice president analyst at Gartner, the barriers to entry for using these tools have significantly lowered. “You don’t have to be an expert to go in and use these tools anymore,” she noted. The automation of complex tasks now allows users to achieve in minutes what once took weeks of coding.
Top Predictive Analytics Tools for 2025
- Altair AI Studio: Known for its strengths in data mining and text mining, Altair AI Studio offers a comprehensive suite of tools for both data scientists and non-coding experts.
- Alteryx AI Platform: This platform excels in automated data preparation and offers robust visual tools for predictive modeling.
- Dataiku: With both visual and code-based interfaces, Dataiku caters to a wide range of users, facilitating data preparation, machine learning, and deployment.
- H2O Driverless AI: This tool simplifies AI development with automated capabilities for feature engineering, model selection, and more.
- IBM Watson Studio: Building on the legacy of SPSS, IBM Watson Studio offers a consolidated platform for various analytics functions.
- Microsoft Azure Machine Learning: Complementing tools like Power BI and Excel, Azure Machine Learning manages the complete predictive analytics lifecycle.
- SAP Analytics Cloud: Integrating BI, planning, and predictive analytics, this tool is ideal for enterprises with extensive SAP deployments.
- SAS: As a pioneer in statistical analytics, SAS continues to innovate with modern data science and machine learning workflows.
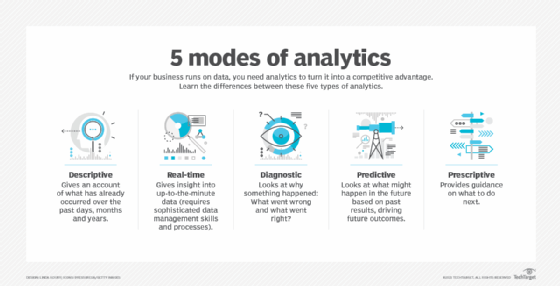
Choosing the Right Tool
When selecting a predictive analytics tool, it’s crucial to align the tool’s capabilities with the specific needs of your organization. Some platforms offer generic solutions applicable across industries, while others provide industry-specific functionalities. Understanding the difference between traditional regression-based tools and machine learning-based tools is also essential for making an informed choice.
Ultimately, the right tool should empower users to collaborate effectively, whether they are seasoned data scientists or business professionals with domain expertise. As businesses continue to integrate AI and machine learning into their workflows, predictive analytics tools will play a pivotal role in driving innovation and success.
Artificial Intelligence Revolutionizing Healthcare: A New Era of Efficiency and Patient Care
Artificial Intelligence Revolutionizing Healthcare: A New Era of Efficiency and Patient Care
The global healthcare industry is on the brink of a transformative shift, as artificial intelligence (AI) technologies are poised to revolutionize patient care and operational efficiency. According to a recent report by GlobeNewswire, the AI in healthcare market is projected to surge at a compound annual growth rate (CAGR) of 18.2% from 2025 to 2035, reaching an estimated value of USD 77,456.4 million by 2035.
Key Drivers of Growth
The exponential growth in the AI healthcare market is driven by several factors. The integration of AI technologies such as machine learning, natural language processing, and deep learning is enhancing diagnostics, treatment planning, and patient management. These advancements are not only streamlining operations but also paving the way for personalized and efficient healthcare delivery.The rising healthcare costs and a growing shortage of care providers are further propelling the demand for AI solutions. AI’s ability to analyze vast amounts of medical data, perform clinical diagnoses, and suggest treatment strategies is proving invaluable in reducing operational costs and improving decision-making efficiency.
Opportunities and Challenges
The potential of AI in healthcare is vast, yet several challenges need to be addressed. Data privacy and security remain significant concerns, as does the high cost of implementation. Moreover, the industry faces a skill gap, with a shortage of professionals trained in AI technologies.Ethical considerations also come to the forefront, particularly regarding AI-driven decision-making in critical healthcare scenarios. Sabyasachi Ghosh, Associate Vice President at Future Market Insights, emphasizes the need for innovation and collaboration to overcome these hurdles and fully realize AI’s potential in healthcare.
Regional Insights and Market Leaders
North America leads the charge in AI healthcare adoption, thanks to advancements in healthcare IT infrastructure and supportive regulatory environments. Europe and the Asia Pacific regions are also experiencing significant growth, driven by increasing awareness and demand for AI-based healthcare solutions.Prominent players in the market include Amazon Web Services, DeepMind, IBM, Intel Corporation, Microsoft Corporation, and Siemens Healthcare, among others. These companies are at the forefront of developing innovative AI solutions that are reshaping the healthcare landscape.
For a detailed analysis, the full report on the Artificial Intelligence in Healthcare Market offers comprehensive insights into this rapidly evolving industry.
The Threats Posed by Environmental, Social, and Governance Policies
The Threats Posed by Environmental, Social, and Governance Policies
In a world increasingly dominated by the language and priorities of Environmental, Social, and Governance (ESG) policies, leaders in business, government, and finance are steering society toward a new paradigm. The American Institute for Economic Research (AIER) recently published a comprehensive paper detailing the profound impact of ESG on public and private institutions worldwide.According to AIER, these top-down restrictions, though well-intentioned, are costly and ineffective in addressing perceived and actual social problems. The paper argues that societies thrive when allowed to solve their issues through decentralized experimentation and innovation. ESG’s advocates, however, aim to reshape the world—from the way we travel and heat our homes to the practices and products businesses must prioritize.
The Ideological Underpinnings
ESG policies are rooted in the concept of stakeholder capitalism, which posits that companies have sweeping social responsibilities and are the property of the community rather than shareholders. This philosophy has permeated institutions globally, leading to a push for a “low carbon” economy built on renewable energy and a dramatic redistribution of wealth and power.Yet, the AIER paper highlights several shortcomings of ESG, including its epistemological and ethical issues, conceptual ambiguity, and inefficiency. ESG’s advocates often conflate financial and nonfinancial objectives, advancing a deeply partisan progressive ideology on climate change, pollution, diversity, and LGBTQ+ issues.
Global Influence and Local Impact
The influence of ESG is not limited to the corporate boardroom. In the United States, President Biden’s administration has signed executive orders prioritizing Diversity, Equity, and Inclusion (DEI) and climate-related financial risks, embedding ESG priorities into federal policy. Similarly, states like California and New York have enacted legislation aligning with ESG goals, while others like Texas and Florida have moved to reduce its impact.International Reach
Across the Atlantic, European Union policymakers have long embraced ESG principles, aiming for Europe to be the first continent to reach net-zero carbon emissions. The EU’s Green Deal, European Climate Law, and Sustainable Finance Disclosures Regulation exemplify the widespread adoption of ESG policies in Europe.Challenges and Criticisms
The AIER paper raises concerns about the transparency and effectiveness of ESG initiatives. Critics argue that ESG criteria often lack clarity and consistency, making it difficult to measure their success. Additionally, the economic costs of ESG are significant, with companies diverting resources to meet compliance requirements rather than focusing on innovation and productivity.As ESG continues to shape the global economy, the debate over its merits and drawbacks persists. The AIER paper serves as a cautionary tale, urging stakeholders to consider the broader implications of ESG policies and their potential to undermine freedom, political self-determination, and economic prosperity.
Revolutionizing Medical Training with Virtual Reality
Revolutionizing Medical Training with Virtual Reality
In the rapidly evolving field of medical education, a groundbreaking study has emerged, shedding light on the transformative potential of immersive technologies. Published on September 9, 2024, in BMC Medical Education, this research explores the role of extended reality (XR) and virtual reality (VR) in training young healthcare professionals to manage medical emergencies more effectively.Addressing a Critical Gap Newly minted healthcare professionals often find themselves ill-prepared for the high-stakes environment of emergency medicine. This lack of practical experience can lead to feelings of inadequacy and, more critically, adverse outcomes for patients. The study, involving 529 senior medical students, delves into how XR/VR technologies can bridge this gap by offering realistic, immersive training scenarios.
High Acceptance and Minimal Discomfort The findings reveal a high level of acceptance and engagement among students using XR/VR for emergency medical training. Importantly, incidences of simulation sickness, a common concern with VR, were minimal. The study also noted that the cost of head-mounted displays did not significantly impact the learning experience, making the technology accessible and effective.
Implications for Medical Education The implications of this study are profound. As the medical field continues to embrace technological advancements, XR/VR could become a staple in medical curricula, equipping future physicians with the skills necessary to handle complex emergency scenarios. This aligns with previous insights, such as those from Wei S. and McEvoy MD et al., highlighting the need for enhanced training methods in emergency medicine.
Looking Ahead While the study underscores the potential of XR/VR in medical education, it also calls for further research to fully harness these technologies. The integration of XR/VR into traditional medical training could represent a paradigm shift, with benefits extending beyond emergency medicine.
Further Exploration
For more insights into this transformative study, visit the journal homepage or explore the open access information.Blockchain Technology: Transforming the Real Estate Landscape
Transforming Real Estate Transactions
Alex Lange, vice president of strategy and innovation for the National Association of Realtors in Chicago, predicts a rapid adoption of blockchain technology within the next three to five years. He believes it will facilitate secure transactions, transforming the traditional process of property transfer. This sentiment is echoed by Graham Simmons, co-chair of the business law group at Norris McLaughlin P.A. in Allentown, who notes that Lehigh Valley markets are slowly embracing blockchain, with increasing comfort levels among stakeholders.
Blockchain promises to create an accurate and secure record of transactions, significantly reducing the potential for fraud. Simmons highlights that secondary markets, particularly those dealing with mortgages or mortgage-backed securities, might be among the first to integrate blockchain into their practices. This integration could benefit larger financial institutions and innovative companies in the Lehigh Valley area.
Decentralized Record Keeping
Daniel Jameson, an attorney at Jameson Stone, LLC, suggests that businesses with substantial resources will initially invest in blockchain before it becomes widely accessible. He emphasizes the decentralized nature of blockchain for record keeping, which aligns with the growing convergence of technologies like AI, IoT, and the metaverse. Lange concurs, stating that this technological convergence makes blockchain a viable option for the real estate industry.
However, despite these advancements, challenges remain. While younger generations may readily embrace such technology, many current property buyers might lack the access or willingness to adopt blockchain. Jameson points out that although many property records have already been digitized, facilitating blockchain adoption, the transition requires a shift in mindset.
Blockchain vs. Cryptocurrency
It is essential to distinguish blockchain from cryptocurrency, such as Bitcoin, which remains volatile and not widely accepted for property transactions. Simmons expresses concerns over Bitcoin’s instability and its potential appeal to nefarious actors. Jameson underscores the cautious approach consumers prefer due to real estate’s high stakes, emphasizing that while blockchain holds promise, its integration within existing financial systems will take time.
In summary, blockchain has significant potential to revolutionize real estate through enhanced processes and security. However, broader adoption and integration challenges persist, necessitating a gradual transition to this innovative technology.
2025 Real Estate Trends in South Korea: A Transformative Landscape
2025 Real Estate Trends in South Korea: A Transformative Landscape
In an era defined by rapid change, South Korea’s real estate market is undergoing a transformation that is as dynamic as it is multifaceted. The landscape in 2025 is being reshaped by a confluence of market dynamics, economic impacts, government policies, and technological advancements. These elements are not only guiding the future of real estate but are also pivotal for investors, policymakers, and stakeholders aiming for sustainable growth and stability.Market Dynamics and Economic Impact
As highlighted in the original Global Banking | Finance article, the South Korean government has prioritized affordable housing to address economic inequality and stimulate urban growth. With real estate prices soaring in major cities, policies to cap rental increases and subsidies for developers are in place, aiming to meet 30% of housing needs in areas like Seoul and Busan.Moreover, the demand for suburban living is on the rise, driven by the shift to remote work and a preference for spacious environments. The government’s investment in transportation infrastructure has been crucial in this suburban shift, with new subway lines and express bus routes reducing commuting times.
Technology and Sustainability in Real Estate
The integration of technology into real estate is revolutionizing the market. Smart homes equipped with IoT devices are becoming standard, catering to consumer expectations for convenience and sustainability. The market for these devices is projected to reach $2 billion by 2025, reflecting robust growth.Furthermore, the focus on green urban development aligns with South Korea’s carbon neutrality goals. Projects like the “Smart Green City” initiative are integrating eco-friendly solutions into urban planning, supported by significant government investments.
Stabilization and Investment Trends
Seoul’s housing market, once volatile due to speculative investments, is showing signs of stabilization. Government interventions, such as tighter loan-to-value ratios and property tax adjustments, have moderated property value growth to 3-5% annually.The commercial real estate sector is thriving, supported by the tech industry’s resilience and the e-commerce boom. Areas like Pangyo Techno Valley are witnessing significant investments, underscoring their status as tech innovation hubs.
Policy and Economic Influences
Regulatory measures are central to stabilizing the real estate market, with restrictions on speculative buying and adjustments to property taxes. The Bank of Korea’s monetary policy, including a base interest rate of 3.0%, aims to balance household debt with economic momentum.Despite challenges like a potential supply shortage of new apartments, opportunities abound in regions poised for growth due to planned infrastructure improvements. These developments invite investors to align strategies with emerging trends.
Demographic and Cultural Shifts
South Korea’s demographic landscape is evolving, with an aging population and shifting family dynamics influencing housing preferences. By 2025, nearly 20% of the population will be 65 or older, driving demand for retirement communities and healthcare-integrated facilities.Cultural trends, such as the rise of single-person households, are reshaping housing demands. Urban areas are seeing increased demand for compact, efficient living spaces that accommodate independent lifestyles.
Technological Innovations and Environmental Goals
The rise of PropTech is transforming real estate transactions and management. Digital platforms and blockchain technology are enhancing transparency and security, while AI-driven recommendations expedite property searches.Environmental sustainability remains a priority, with builders incorporating renewable energy and eco-friendly materials into construction. Government incentives promote green building practices, ensuring that over 50% of urban spaces will focus on sustainability by 2030.
The combination of technological innovations and environmental commitments is reshaping South Korea’s real estate market, presenting both opportunities and challenges for stakeholders aiming for future growth.
The AI Revolution in Healthcare: Erez Meltzer’s Vision for the Future
Erez Meltzer, CEO of Nanox, is at the forefront of a groundbreaking transformation in healthcare. With over 35 years of leadership experience, Meltzer is steering the industry into an era where artificial intelligence (AI) is reshaping patient care. This evolution goes beyond mere enhancements; it promises truly individualized healthcare on a grand scale.

The concept of personalized medicine is not new, yet its effective implementation has been hampered by the complexities of human biology and the vast data involved. Enter AI, with its unparalleled computing power and analytic capabilities, capable of processing this complexity to offer meaningful insights. As AI continues to learn from healthcare data, its accuracy and predictive power grow, further enhancing its ability to tailor patient care.
Enhancing Diagnostics and Early Detection
In diagnostics, AI is making remarkable strides. Deep learning models analyze medical imaging data with impressive speed and precision. These AI systems are not replacing radiologists but augmenting their capabilities, leading to more precise diagnoses and the quick identification of incidental findings.
AI’s real strength lies in personalizing the diagnostic process. By considering individual risk factors, AI can tailor screening schedules, ensuring high-risk patients receive more frequent screenings while minimizing unnecessary procedures for low-risk individuals. This approach not only improves patient outcomes but also optimizes healthcare resources.
Predictive Analytics: A New Frontier in Preventive Care
AI’s potential in predictive analytics is vast. By integrating data from diverse sources, such as electronic health records and genetic information, AI models can predict individual patient risks with unprecedented accuracy. For instance, researchers at the University of Virginia have developed an AI model for predicting outcomes in heart failure patients, offering personalized risk assessments that enable tailored interventions.
Similarly, a pancreatic cancer risk model developed at MIT can potentially expand the group of patients who benefit from early screening. Such predictive capabilities pave the way for proactive care strategies, reducing chronic disease burdens and improving overall health outcomes.
Personalizing Treatment Plans
AI’s impact extends into treatment planning. A team at Northwestern University’s McGaw Medical Center is working on a model to predict long-term outcomes for breast cancer patients. This model aims to help pathologists recategorize patients, allowing for shorter, less intense treatment plans with fewer side effects, marking a significant advancement in personalized cancer treatment.
Addressing Challenges and Ethical Considerations
Despite AI’s promise in healthcare, challenges remain. Institutional complexity and potential biases in AI models are significant hurdles. Ensuring AI-driven healthcare is fair and equitable requires ongoing attention to diverse data sets and the adaptability of AI algorithms. As AI becomes more integral to healthcare decisions, addressing these challenges is paramount to maintaining patient trust and improving outcomes.
The Path Forward
Looking ahead, AI holds the promise of revolutionizing healthcare by enabling personalization across the patient journey. From early detection to treatment planning, AI can help create a more effective, efficient, and patient-centered healthcare system. However, it’s crucial to remember that AI is a tool to support healthcare professionals, not replace them.
As we continue to develop AI technologies, we must do so responsibly, focusing on improving patient outcomes and maintaining trust. The future of healthcare is personalized, predictive, and proactive, and by embracing these technologies thoughtfully, we can work toward a system that truly centers on the individual patient.
The AI revolution in healthcare is well underway. As industry leaders, it’s our responsibility to guide this transformation, ensuring that we harness the power of AI to create a healthcare system that serves all patients better. The potential benefits—lives improved and saved—are too significant to ignore.
Predictive Analytics Tools in 2025: Revolutionizing Business Intelligence
Revolutionizing Business Intelligence
Predictive analytics has undergone a significant transformation. Once the domain of specialized data scientists, advancements in AI have democratized these tools, making them user-friendly even for those without technical expertise. As TechTarget reports, this shift is driven by a need to simplify complex analytics processes and integrate machine learning models into business workflows.
Top Tools for 2025
In its recent feature, TechTarget profiles eight leading predictive analytics tools that are setting the stage for 2025:- Altair AI Studio: Known for its data mining capabilities, it simplifies data extraction and modeling workflows.
- Alteryx AI Platform: Offers automated data preparation and integration with other ML platforms.
- Dataiku: Provides a platform for both technical and non-technical users to generate insights.
- H2O Driverless AI: Focuses on automation in AI development, making it accessible to all.
- IBM Watson Studio: Integrates predictive analytics with a broad range of functions, enhancing collaboration.
- Microsoft Azure Machine Learning: Complements its core tools with lifecycle management for predictive analytics.
- SAP Analytics Cloud: Combines BI, planning, and predictive analytics into a unified suite.
- SAS: Continues its legacy with modernized data science and machine learning workflows.
The Democratization of Analytics
Carlie Idoine of Gartner highlights the growing accessibility of these tools, noting that automation has reduced the need for deep technical expertise. This democratization is further evidenced by platforms like Tableau’s integration with Einstein Discovery, which empowers business users with AI-driven insights.Looking Ahead
As businesses prepare for the future, the integration of predictive analytics tools into everyday operations is crucial. The insights from TechTarget underscore the importance of selecting the right tools to meet diverse business needs, from lead scoring to fraud reduction.In conclusion, the landscape of predictive analytics is poised for continued growth and innovation. By leveraging these tools, businesses can not only gain a competitive edge but also drive informed decision-making processes across all levels.
Navigating TikTok: A Realtor’s Guide to Success
So, You’re a Realtor and Want to Crush It on TikTok? Here’s What You Need to Know
Ever wondered how TikTok became the playground for realtors? I mean, who would’ve thought that a platform famous for dance challenges and lip-syncing could double as a powerhouse lead-generation tool? If you’re not on TikTok yet as a real estate agent, what are you even doing? Seriously, the market is there, it’s free, and it’s begging for you to shine. But don’t panic if you’re starting from scratch—today, we’re diving into Karis’s masterclass on the TikTok basics for realtors. Spoiler: It’s the step-by-step guide you didn’t know you needed.
Setting the Stage—Why TikTok?
Let’s address the elephant in the room—TikTok, for realtors? Yes, absolutely! Karis kicks off the conversation by acknowledging a common struggle: so many agents know they should be on TikTok but have zero clue where to begin. And hey, no shame—jumping into a new app can feel like showing up to an open house in your pajamas.
TikTok isn’t just for teens or viral dances anymore. It’s proven itself as a legit tool for branding and lead generation for realtors. Karis herself says that TikTok is the top source for her business, and she’s not gatekeeping her playbook.
A Realtor’s TikTok Starter Pack
Okay, so imagine you’re downloading TikTok for the very first time—no filters, no followers, just you and the untapped power of your potential. Here’s the breakdown straight from Karis:
- Claim Your Identity (Name + Username): Your username is searchable—huge win! Stick with your full name or add “Realtor” if you’re building a personal brand. If you rep a team, branding cohesion is key.
- Keep It Cohesive (Profile Picture): This isn’t a selfie free-for-all. Karis recommends using the same profile picture across all platforms (Instagram, LinkedIn, Google My Business). Why? Because consistency builds trust, and people recognize your face.
- Craft a Killer Bio: Two MUST-haves in there:
- Mention you’re a realtor or real estate agent.
- Include your location (state, city, or region).
- Don’t skimp on adding a website! Karis suggests using a tool like Linktree for bonus efficiency. Why list one link when you can subtly flex all your platforms in one neat package? Genius.
TikTok Navigation—Master the Playground
Navigating TikTok as a newbie can feel like wandering into IKEA—it’s overwhelming, and everything looks fun. Karis demystifies it step by step:
- Your Home Page has two sections: the Following tab (for accounts you follow) and the For You page (where the algorithm gets witchy and shows you curated content). Initialize your feed by following other top creators in your niche—hello, instant inspo!
- The Discover Page is where trends, sounds, and hashtags live. Use it to spy on what’s hot in your area and niche. Think of it like networking at a conference—but you’re in your pajamas.
- Drafts are Your Best Friend: Batch content creation, people! Spend an hour pumping out content, save it to drafts, and drip-feed it later. This is TikTok 101 for busy professionals.
A Crash Course in Video Creation
Now for the crux of it all: making your first TikTok. Karis turns this potentially terrifying feat into something as straightforward as assembling a pre-cut charcuterie board.
- Set your video timer to 60 seconds. Not only does this keep things concise, but it also makes your video repurposable for Instagram Reels and YouTube Shorts. Multitasking for the win!
- Use tripods and timers to shoot hands-free (and avoid the dreaded shaky camera look).
- Add captions and hashtags: Create context for your audience by detailing what your video is about and using a sprinkle of niche-specific hashtags (3–5 is perfect).
- Pin your BEST videos: Highlight your top three performing TikToks on your profile to make your page binge-worthy for new visitors.
But Why Does the TikTok Algorithm Feel Like a Magic Eight Ball?
TikTok isn’t pulling content out of a hat; it’s running on a laser-focused algorithm that learns your preferences as you engage. Karis explains it brilliantly: your early posts will feel like dropping a pebble into a massive pond, but as the algorithm analyzes user behavior (views, likes, shares), it’ll start showing your content to more people who might care.
Want to find top-performing realtors or trends in your area? Search for them. Want TikTok to know you’re into real estate content? Binge-watch a few related videos. The app learns what you like—and what your future followers will like, too.
TikTok Is More Than Fun—it’s a Legit Business Tool
Now, here’s where Karis really drives it home: TikTok is free. If you’re not leveraging a platform that can send you high-quality leads while making entertaining content, are you even hustling? (Harsh, but true.) Not to mention, TikTok gives you that sweet opportunity to connect with people not just as a salesperson but as, you know, you.
Karis suggests involving TikTok in your overall real estate strategy and seeing where it takes you. Why wouldn’t you want to do something that’s equal parts fun and lucrative?
Final Thoughts: The TikTok Realtor Revival is Here
Karis delivers a goldmine of tips for TikTok noobs, but honestly, her advice transcends the real estate niche. Whether you’re a realtor, a small business owner, or just somebody trying to get your face on the digital map, there’s something valuable here for everyone.
So, let’s pass the mic to you: Have you started creating TikTok content yet? Did you try any of Karis’s tips already? Let’s talk in the comments because, let’s face it, we’re all just here trying to figure out this wacky little app together.
Your move, realtors! Hit me up with your stories or even drop your TikTok handle below—I could always use some niche-specific inspo myself. Who needs another scrolling binge when we could be scrolling you?
Why Toronto’s Real Estate Market Is a Rollercoaster Using Would You Rather Decisions
Why Toronto’s Real Estate Market Is Basically Playing ‘Would You Rather?’
Ever find yourself scrolling through charts and stats about real estate prices and think, “This looks like my retirement plan—confusing and kind of worrisome?” Well, buckle up, because Toronto’s real estate market has been on a rollercoaster ride that makes Canada’s Wonderland look like a kids’ playground. And honestly, it’s giving me some serious “Do I laugh or cry?” vibes.
The Great Toronto Housing Drama You Missed
Let’s get everyone up to speed with some context, shall we? Picture this: in 2020, the average home price in the Greater Toronto Area (GTA) sat at a (still crazy) $930,000. Then came the pandemic—a time when sourdough bread rose, TikTok dances went viral, and apparently, home prices decided to hit turbo mode. By 2022, the average cost had skyrocketed to $1.19 million. That’s, like, a 28% increase in just three years!
But as with all wild parties, there was an inevitable hangover. Cue the economic chaos, spiking interest rates, and “what-is-happening-right-now” vibes in the market. By the end of 2024, everything sort of… plateaued? The average price dipped just slightly to $1.126 million, which, let’s be honest, is still an astronomical price tag. Compared to the peak, that’s only a 5.3% drop. Let’s pause for a moment of silence for my very not-millionaire-level bank account.
The Big Question: Up or Down in 2025?
Okay, so here’s where things get spicy. Are we gearing up for another housing boom, or should we brace for more tears (and maybe some very serious calls to our landlords)? That depends on who you ask.
- On the bullish side: Big banks like TD are forecasting a 6.4% increase in prices by 2025. Now, I’m no economist, but I do know a suspiciously optimistic prediction when I see one.
- On the bearish side: Remember when Toronto’s housing market imploded in the late ’80s? Prices didn’t just drop—they spent seven years in the real estate penalty box before beginning a snail-paced recovery.
Mark Twain’s famous words, “History doesn’t repeat, but it often rhymes,” never felt more relevant.
My Personal Take: This Is Like Predicting the Oscars
You know that moment when you’re watching the Oscars, and you’re absolutely sure your favorite movie will win Best Picture? And then they call something completely unexpected, and you’re left shaking your popcorn bag in disbelief? Yeah, that’s how it feels trying to guess Toronto’s housing market right now.
- Freehold Properties: These are the prom king and queen of the housing world. Everyone loves them, they’re in demand, and they perform better (looking at you, townhomes and semi-detached).
- Condos: Sorry, condos, but you’re kind of like the reliable but boring friend in the GTA real estate market. You’re staying flat while everyone else is out here stealing the show.
If I had to throw my hat in the ring, I’d go with a more modest prediction. Maybe we’re looking at a small increase (2.5% max if rates drop—big if there) or relative stability. A major boom? Hard to imagine unless the Bank of Canada busts a move with those interest rates. At the same time, I don’t see prices absolutely tanking either.
Analysis: Is Real Estate Still the Move?
Here’s the truth, friends: deciding if you should keep chasing property ownership in Toronto is like trying to figure out if you should bet on Teslas because Elon Musk tweets funny memes. The transcript draws a great parallel here: just like you can’t judge Tesla stock by looking at its did-you-just-skyrocket chart, you can’t judge Toronto’s housing market just by glancing at price tags.
Context matters. Nuances matter. And, let’s be real, timing really, really matters.
Let’s Chat! What’s Your Move?
So, what do YOU think? Are we in for a 1980s-style market meltdown or another era of climbing prices and sad PayPal balances? Would you buy now or hold out for a theoretical crash? And seriously, if someone actually predicts 2025 prices correctly, can we name them the real estate Oracle of Toronto?
Drop your thoughts in the comments (or, you know, just yell them into the void—you do you). In the meantime, I’ll be over here plotting my next move… or at least trying to figure out if I should switch to buying plants instead of real estate. Less commitment, more green vibes.
TikTok’s Ban: US Social Media Frenzy
TikTok’s Ban Has Everyone Spiraling—Here’s What You Need to Know (and Why It’s Hilarious)
Ever wondered what happens when you try to take away the internet’s favorite toy? Spoiler alert: chaos. Absolute chaos.What Just Happened?
Okay, quick context for those out of the loop: TikTok, beloved by 200 million U.S. users, has officially been yeeted off the app store because, long story short, its Chinese parent company, ByteDance, wouldn’t stop sharing user data with Beijing. And when I say they wouldn’t stop, I mean this was like your mom saying, “I better not catch you sneaking cookies again,” and then finding you elbow-deep in the cookie jar 15 minutes later. The U.S. government had repeatedly warned them, and finally said, “Alright, we’re done. Say goodbye to your favorite distraction.” Now, let’s get this straight—this isn’t necessarily a “free speech” issue, even though some folks on the conservative side are spinning it that way. The government isn’t trying to shut people up; they just wish ByteDance would stop handing over our memes, dance tutorials, and search histories to another country.But do I think banning TikTok outright is the best move? Not really. I get the whole “data security” thing is serious, but let’s be honest: Facebook and Instagram are ALSO snooping through your life like the nosiest neighbor on the block.
The Reactions: Armageddon or Comedy Gold?
Oh man, the drama. Between influencers crying about losing their “safe space” and others live blogging the “death of culture,” I am living for the TikTok ban’s content (ironically). One guy tearfully posted, comparing the ban to parents yanking him out of school mid-year and saying goodbye to all his friends. Bro, it’s an app, not a childhood dog.- Some people are comparing it to losing a job.
- Others are dismissing it as just “content creators whining.”
- Meanwhile, meme-makers are thriving over the chaos.
Is This Just Another Trend?
Now, let’s zoom out for a second—is the TikTok ban a one-time, big-time event, or is this another sign of the shift in how we think about data privacy? Remember when Donald Trump also flirted with banning TikTok, before doing a total 180 by proposing a fun little idea of the U.S. owning 50% of the app? Well, it seems like we’ve hit the sequel, but without the promise of dramatic Trumpian executive orders to#SaveTikTok.
And let’s not forget this might be the start of a multi-platform domino effect. If TikTok is toast, Instagram Reels, YouTube Shorts, or bizarre TikTok lookalikes (looking at you, Lemon8) are poised to swoop in and capitalize.
What’s Next for Us, the TikTok-Orphans?
If you’re in the “I need my short-form hit of dopamine” camp, don’t panic just yet. TikTok isn’t down-for-the-count forever—or at least, that seems unlikely. Let’s give it a week, and I wouldn’t be shocked if it strikes some great big deal to “fix its issues” (translation: sell part of the company). In the meantime, creators are urging folks to follow them on other platforms. Everyone’s practically screaming, “Follow me on Instagram Reels! It’s basically the same thing, only clunkier!”Sutter Health’s Innovative Approach to Sepsis Management
Sutter Health’s Innovative Approach to Sepsis Management
Sutter Health is pioneering a transformative approach in critical care by introducing the FloPatch, a novel wearable device aimed at enhancing clinical decision-making. This initiative focuses on improving sepsis management within intensive care units (ICUs), leveraging real-time data to support critical treatment decisions. Sepsis, a severe condition marked by an extreme response to infection, affects approximately 1.7 million American adults each year, often resulting in significant mortality. Sutter Health’s deployment of the FloPatch device aims to refine fluid management protocols, which are crucial for precise sepsis treatment.
Sepsis, a severe condition marked by an extreme response to infection, affects approximately 1.7 million American adults each year, often resulting in significant mortality. Sutter Health’s deployment of the FloPatch device aims to refine fluid management protocols, which are crucial for precise sepsis treatment.



































